Visible light-induced intramolecular cyclization reactions of diamines: a new strategy to construct tetrahydroimidazoles†
Abstract
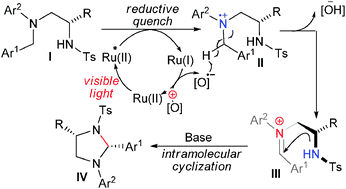
A new and efficient synthesis of highly substituted tetrahydroimidazole derivatives by means of visible light-induced intramolecular cyclization reactions has been described. This photoredox catalytic reaction exhibited high diastereoselectivity and afforded the desired products in good yields.

 Please wait while we load your content...
Please wait while we load your content...