Electrochemical evidence for catalyticwater oxidation mediated by a high-valent cobalt complex†
Abstract
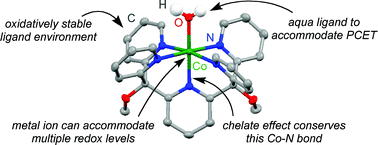
The pH-dependent electrochemical behavior for a Co(II) complex, [Co(Py5)(OH2)](ClO4)2 (1; Py5 = 2,6-(bis(bis-2-pyridyl)methoxymethane)pyridine), indicates consecutive (

 Please wait while we load your content...
Please wait while we load your content...