Substituent and counterion effects on the formation of π-dimer dications of end-capped heptathienoacenes†‡
Abstract
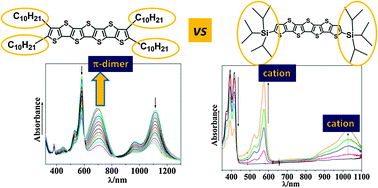
We have investigated the impact of the functionalization and the chemical nature of counterions on the π-dimer dications formation in two end-capped heptathienoacenes. Radical

 Please wait while we load your content...
Please wait while we load your content...