Highly enantioselective recognition of a wide range of carboxylic acids based on enantioselectively aggregation-induced emission†
Abstract
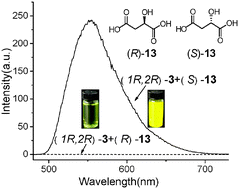
A new chiral fluorescence receptor, designed to exploit aggregation-induced emission (AIE), exhibited not only an exceptionally high enantioselectivity but was also applicable to a wide range of chiral

 Please wait while we load your content...
Please wait while we load your content...