Acyl hydrazides as peptoid sub-monomers†
Abstract
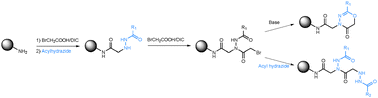
The use of acyl hydrazides as peptoid sub-monomers is investigated. We demonstrate here that azapeptoids derived entirely from acyl hydrazides can be made conveniently and efficiently using standard peptoid sub-monomer chemistry. Structural studies reveal that the main chain

 Please wait while we load your content...
Please wait while we load your content...