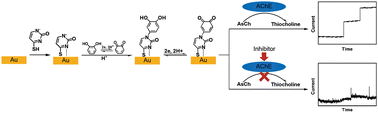
An electrochemical platform for acetylcholinesterase (AChE) activity assay and its inhibitors screening is developed based on the Michael addition reaction of thiocholine, the hydrolysis product of acetylthiocholine (AsCh) in the presence of AChE, with the electrogenerated o-quinone of catechol-terminated SAMs on a gold electrode. For understanding and confirming the mechanism of the reaction, the electrochemical behaviors of Michael addition reaction of two model compounds, cysteine (CYS) and glutathione (GSH), towards the catechol-terminated SAMs have been studied. The enzyme kinetics and the inhibition effects of three types of AChE inhibitors, which are tacrine, carbofuran and parathion-methyl, have been investigated using an amperometric method. Among these three inhibitors, tacrine exhibits the strongest inhibiting effect, which is reinforced by the resulting data of kinetic studies on each inhibitor's influence upon the enzyme activity.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...