Electrochemical evidences and consequences of significant differences in ions diffusion rate in polyacrylate-based ion-selective membranes
Abstract
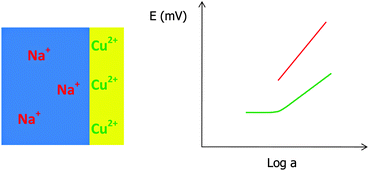
The origin and effect of surface accumulation of primary ions within the ion-selective poly(n-butyl acrylate)-based membrane, obtained by thermal

 Please wait while we load your content...
Please wait while we load your content...