A novel method for ranitidine hydrochloride determination in aqueous solution based on fluorescence quenching of functionalised CdS QDs through photoinduced charge transfer process: Spectroscopic approach
Abstract
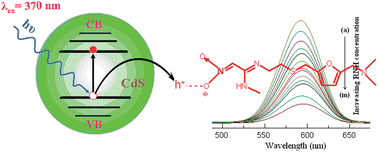
A novel method for the quantitative determination of

 Please wait while we load your content...
Please wait while we load your content...