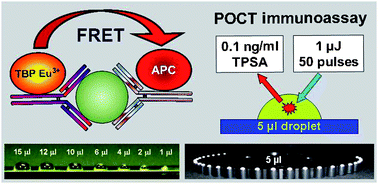
Toward sensitive, quantitative point-of-care testing (POCT) of protein markers: miniaturization of a homogeneous time-resolved fluoroimmunoassay for prostate-specific antigen detection†
Abstract
Point-of-care testing (POCT) systems which allow for a sensitive, quantitative detection of

 Please wait while we load your content...
Please wait while we load your content...