Site-selective chemoenzymatic construction of synthetic glycoproteins using endoglycosidases†
Abstract
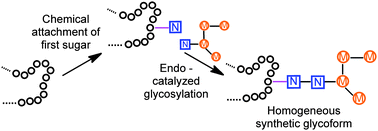
Combined chemical tagging followed by Endo-A catalysed elongation allows access to homogeneous, elaborated glycoproteins. A survey of different linkages and

 Please wait while we load your content...
Please wait while we load your content...