Visualization of the interaction between Gβγ and tubulin during light-induced cell elongation of Blepharisma japonicum
Abstract
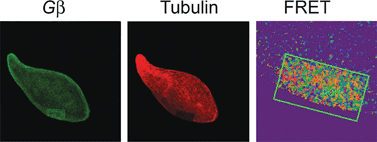
Blepharisma japonicum ciliates display reversible cell elongation in response to lasting bright illumination. This light-induced phenomenon has been ascribed to the active sliding of the cortical microtubules of the ciliate. The detailed intracellular signaling pathway that activates the microtubule network in response to light, resulting in cell elongation, is unknown. We have previously reported that light stimulation initiates sequential molecular events consisting of a decrease in the phosphorylation of ciliate Pdc, followed by increased binding of Pdc to membrane-localised Gβγ and the subsequent translocation of the Pdc-Gβγ complex to the cytoplasm. In this study, we used selected agents known to influence protein phosphorylation to test whether alterations in Pdc phosphorylation levels by light affect ciliate shape. Behavioural analysis indicated that cell treatment with okadaic acid, an inhibitor of protein phosphatase activity, heavily abolished the effect of light on cell elongation, whereas the presence of H-89, a specific inhibitor of cAMP-dependent protein kinase (PKA) activity, had no appreciable effect on the cell length. Phosphorylation assays showed that cell incubation with H-89 mimicked light by promoting Pdc dephosphorylation and its colocalization with Gβγ. However, as demonstrated by FRET-AP, Pdc-Gβγ complex formation and changes in the length of the cell did not occur under the same conditions. Moreover, fluorescence microscopy showed localization of Gβγ and β-tubulin in the same cell compartment and demonstrated that a direct interaction between these proteins occurs in cells adapted to darkness or exposed to prolonged illumination (≥10 min). In contrast, an opposite effect, i.e. a transient decrease in the interaction between Gβγ and β-tubulin and distinct Pdc dephosphorylation, was observed in cells illuminated for short time. Under these conditions, Pdc preferentially occupies the cell submembrane region and interacts with Gβγ. In cells illuminated for a longer time (≥10 min) and despite the constant light intensity, Pdc was progressively rephosphorylated and then dissociated from Gβγ, relocalizing within the cell cytoplasm. The results obtained in this study suggest that alterations in Pdc phosphorylation may be involved in light-induced elongation of the Blepharisma cell body, which affects the interaction of Gβγ with β-tubulin and cell cytoskeleton remodelling.

 Please wait while we load your content...
Please wait while we load your content...