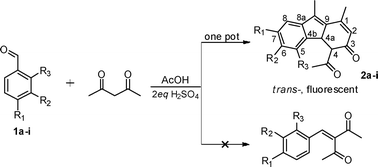
New fluorescent trans-dihydrofluoren-3-ones from aldol–Robinson annulation–regioselective addition involved one-pot reaction†
Abstract
An unexpected discovery of new trans-4-acetyl-1,9-dimethyl-4,4a-dihydro-3H-fluoren-3-ones from one pot reactions of benzaldehydes and

 Please wait while we load your content...
Please wait while we load your content...