A three-step tandem process for the synthesis of bicyclic γ-lactams†
Abstract
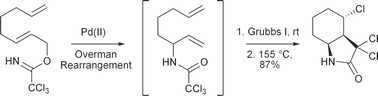
A one-pot, three-step tandem process has been developed for the direct synthesis of functionalised bicyclic [3.3.0], [4.3.0] and [5.3.0] γ-lactams from simple allylic trichloroacetimidates. The process involves a
 Please wait while we load your content...
Please wait while we load your content...