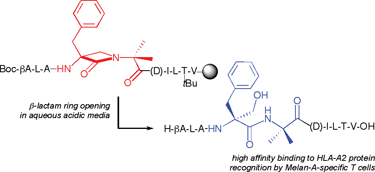
Design, synthesis and evaluation of β-lactam antigenic peptide hybrids; unusual opening of the β-lactam ring in acidic media†
Abstract
β-Lactam
* Corresponding authors
a
Université de Bordeaux, Institut des Sciences Moléculaires (UMR-CNRS 5255) and Institut Européen de Chimie et Biologie (IECB), 2 rue Robert Escarpit, France
E-mail:
s.quideau@iecb.u-bordeaux.fr
Fax: (+33) 5-4000-2215
Tel: (+33) 5-4000-3010
b Departamento de Química Orgánica-I, Universidad del País Vasco, Joxe Mari Korta R&D Center, Apdo. Tolosa-72, Spain
c Department of Medical Biochemistry and Immunology, Henry Wellcome Building, Cardiff University School of Medicine, Heath Park, Cardiff, UK
d Department of Chemistry and Biochemistry, 251 Nieuwland Science Hall, University of Notre Dame, Notre Dame, USA
β-Lactam

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. Tarbe, I. Azcune, E. Balentová, J. J. Miles, E. E. Edwards, K. M. Miles, P. Do, B. M. Baker, A. K. Sewell, J. M. Aizpurua, C. Douat-Casassus and S. Quideau, Org. Biomol. Chem., 2010, 8, 5345 DOI: 10.1039/C003877F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
