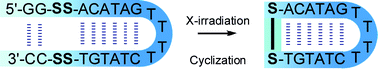
Radiolytic cyclization of stem-and-loop structured oligodeoxynucleotide with neighboring arrangement of α,ω-bis-disulfides†
Abstract
Upon X-ray irradiation of hypoxic aqueous solution, modified oligodeoxynucleotides (ODNs) bearing a pair of disulfides at both ends of the strand that forms a stem-and-loop structure with a neighboring arrangement of α,ω-bis-disulfides underwent efficient
 Please wait while we load your content...
Please wait while we load your content...