Synthesis and biological evaluation of new penta- and heptacyclic indolo- and quinolinocarbazole ring systems obtained viaPd0 catalysed reductive N-heteroannulation†
Abstract
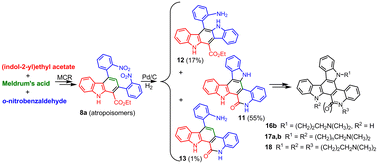
A short route, involving a tetramolecular
* Corresponding authors
a
Université de Reims-Champagne-Ardenne, Institut de Chimie Moléculaire de Reims, CNRS UMR 6229, Groupe BSMA, UFR de Pharmacie, 51 rue Cognacq-Jay, Cedex, France
E-mail:
janos.sapi@univ-reims.fr
Fax: +33(0)326918029
b Laboratoire de Pharmacologie Antitumorale, INSERM U-837, Centre Oscar Lambret, Université Nord de France, Institut de Recherches sur le Cancer de Lille, IMPRT, 1 Place de Verdun, Cedex, France
c Université de Reims-Champagne-Ardenne, CNRS UMR 6237, IFR 53 Biomolécules, UFR des Sciences Exactes et Naturelles, BP 1039, Cedex 2, France
d Université de Picardie Jules Verne, Laboratoire des Glucides, CNRS UMR 6219, Equipe THERA, Faculté de Pharmacie, 1 rue des Louvels, Cedex 1, France
e Université de Reims-Champagne-Ardenne, Institut de Chimie Moléculaire de Reims, CNRS UMR 6229, Groupe IS, UFR des Sciences Exactes et Naturelles, Bat. 18., BP 1039, Cedex 2, France
f Université Paris Descartes, Synthèse et Structure de Molécules d'Intérêt Pharmacologique, CNRS UMR 8638, Faculté des Sciences Pharmaceutiques et Biologiques, 4 avenue de l′Observatoire, Cedex 06, France
g Université de Lille 2, EA 4034, Laboratoire de Chimie Analytique, Faculté des Sciences Pharmaceutiques et Biologiques, 3 rue du Professeur Laguesse, BP 83, Cedex, France
h CNRS, Protein Phosphorylation & Human Disease Group, USR3151, Station Biologique, BP 74, France
i Régulation et Dynamique des Génomes, INSERM U565, CNRS UMR 5153, USM 503, Muséum National d'Histoire Naturelle, 43 rue Cuvier, CP26, Cedex 5, France
j
Université de Reims-Champagne-Ardenne, Institut de Chimie Moléculaire de Reims, CNRS UMR 6229, Groupe BSMA, UFR des Sciences Exactes et Naturelles, Bat. 18., BP 1039, Cedex 2, France
E-mail:
eric.henon@univ-reims.fr
Fax: +33(0)326918497
A short route, involving a tetramolecular

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
M. Laronze-Cochard, F. Cochard, E. Daras, A. Lansiaux, B. Brassart, E. Vanquelef, E. Prost, J. Nuzillard, B. Baldeyrou, J. Goosens, O. Lozach, L. Meijer, J. Riou, E. Henon and J. Sapi, Org. Biomol. Chem., 2010, 8, 4625 DOI: 10.1039/C0OB00149J
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
