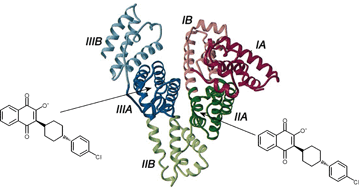
High-affinity human serum albumin (HSA) binding of the C3-substituted antimalarial 2-hydroxy-1,4-naphthoquinone derivative atovaquone (ATQ) has been demonstrated and studied by circular dichroism (CD), UV/VIS absorption, fluorescence spectroscopy and affinity chromatography methods. The analysis of induced CD data generated upon HSA binding of ATQ revealed two high-affinity binding sites (Ka ≈ 2 × 106 M−1). CD interaction studies and displacement of specific fluorescent and radioactive marker ligands indicated the contribution of both principal drug binding sites of HSA to complexation of ATQ, and also suggested the possibility of simultaneous binding of ATQ and some other drugs (e.g. warfarin, phenylbutazone, diazepam). Comparison of UV/VIS spectra of ATQ measured in aqueous solutions indicated the prevalence of the anionic species formed by dissociation of the 2-hydroxyl group. HSA binding of related natural hydroxynaphthoquinones, lapachol and lawsone also induces similar CD spectra. The much weaker binding affinity of lawsone (Ka ≈ 104 M−1) bearing no C3 substituent highlights the importance of hydrophobic interactions in the strong HSA binding of ATQ and lapachol. Since neither drug exhibited significant binding to serum α1-acid glycoprotein, HSA must be the principal plasma protein for the binding and transportation of 2-hydroxy-1,4-naphthoquinone-type compounds which are ionized at physiological pH values.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...