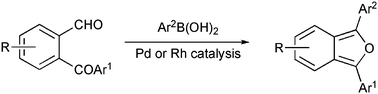
Variously substituted 1,3-diarylisobenzofurans have been regiospecifically prepared via palladium- and rhodium-catalysed reaction of functionalised boronic acids onto o-acylbenzaldehydes. Rhodium catalysis has furthermore been improved using microwave activation. Thus, isobenzofurans containing aryl groups substituted by halogens, unprotected amine, alcohol and even aldehyde groups, have been obtained directly in good to satisfactory yields. Divergent results have been observed when palladium-, rhodium- and MW-activated rhodium-catalysis was applied to the reaction of phenylboronic acid with an iodinated o-acylbenzaldehyde, leading principally to Suzuki coupling product and/or to iodinated isobenzofuran.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Please wait while we load your content...
Please wait while we load your content...