Regioselective synthesis of 3-acylindolizines and benzo- analogues via1,3-dipolar cycloadditions of N-ylides with maleic anhydride†
Abstract
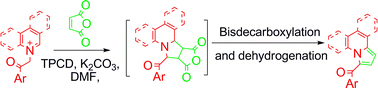
3-Acylindolizines (5a–5f) and their benzo- analogues 1-acylpyrrolo[1,2-a]quinolines (6a–6f) and 1-acylpyrrolo[2,1-a]isoquinolines (7a–7i) are regioselectively synthesized by a convenient one pot reaction of the corresponding pyridinium (quinolinium, isoquinolinium)

 Please wait while we load your content...
Please wait while we load your content...