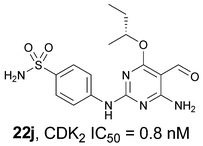
CDK2 inhibitory structure–activity relationships have been explored for a range of 5-substituted O4-alkylpyrimidines. Variation of the 5-substituent in the 2,6-diaminopyrimidine series confirmed the 5-nitroso substituent as optimal, and showed that 5-formyl and 5-acetyl substituents were also tolerated at this position. A series of O4-alkyl-N2-aryl-5-substituted-6-aminopyrimidines revealed interesting structure–activity relationships. In the 5-nitroso series, the optimum O4-alkyl substituents were cyclohexylmethyl or sec-butyl, combined with a 2-sulfanilyl group. By contrast, in the N2-arylsulfonamido-5-formyl series, the cyclohexylmethyl compound showed relatively poor activity compared with the sec-butyl derivative (22j, (R)-4-(4-amino-6-sec-butoxy-5-formylpyrimidin-2-ylamino)benzenesulfonamide; CDK2 IC50 = 0.8 nM). Similarly, in the N2-arylsulfonamido-5-(hydroxyiminomethyl) series the O4-sec-butyl substituent conferred greater potency than the cyclohexylmethyl (23c, (rac)-4-(4-amino-6-sec-butoxy-5-(hydroxyiminomethyl)pyrimidin-2-ylamino)benzenesulfonamide; CDK2 IC50 = 7.4 nM). The 5-formyl derivatives show selectivity for CDK2 over other CDK family members, and are growth inhibitory in tumour cells (e.g.22j, GI50 = 0.57 μM).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...