Biocatalysed concurrent production of enantioenriched compounds through parallel interconnected kinetic asymmetric transformations†‡
Abstract
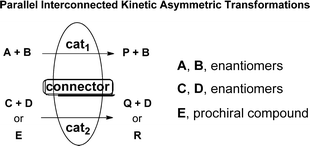
Parallel interconnected kinetic asymmetric transformations were performed in order to obtain enantioenriched derivatives starting from a set of racemic or prochiral compounds. Thus, in a one-pot reaction using two

 Please wait while we load your content...
Please wait while we load your content...