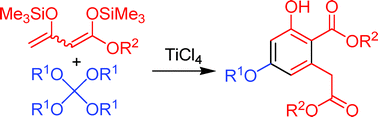
Synthesis of 3-hydroxy-5-alkoxyhomophthalates by domino ‘2 : 1-coupling/intramolecular aldol condensation’ reactions of 1,3-bis(trimethylsilyloxy)-1,3-butadienes with tetraalkoxymethanes†
Abstract
The first domino ‘2 : 1 condensation/intramolecular aldol’ reactions of

 Please wait while we load your content...
Please wait while we load your content...