A stereoselective intramolecular cyclopropanation via a de novo class of push–pull carbenes derived from DMDO-epoxidations of chiral ynamides†
Abstract
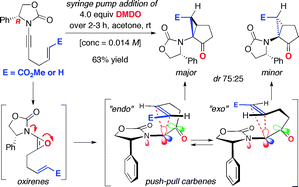
This work describes the first examples of diastereoselective intramolecular cyclopropanations of a de novo class of push–pull carbenes derived from DMDO-epoxidations of chiral ynamides. This reaction sequence essentially constitutes a tandem

 Please wait while we load your content...
Please wait while we load your content...