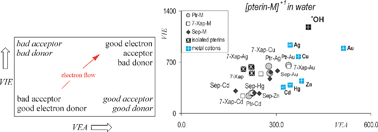
Many animal colorants have been described as scavengers of free radicals. Pterins, also known as pteridines, represent one of these pigments. These molecules are analogous to guanine and consist of heterocyclic compounds, which have the highest nitrogen content of any colorant analyzed from animals. One of the mechanisms used for scavenging free radicals is that of the electron transfer reaction, which can be analyzed in terms of the electron donor–acceptor properties of these molecules. The interaction of metal atoms with pterins modifies the properties of pterins, in such a way that they manifest altered electron transfer properties. In this paper, Density Functional Approximation calculations are used to analyze the interaction of pterins (pterin, isoxanthopterin and sepiapterin) with metal atoms (M = Cu, Ag, Au, Zn, Cd or Hg). Neutral (in gas phase), cations and di-cations (in water) are analyzed in order to assess the effect of the positive charge in the M–pterin interaction. Evidently, there is a correlation between the dissociation energy involved in the removal of the metal atom and the ionization energy of the metal atom. Those pterins containing certain cationic metals (such as Zn, Cd and Hg) are depicted as better antioxidants than other pterins, and likewise the interaction with Cu, Ag and Au (di-cations) produces compounds that may act as electron acceptors. The electron transfer reaction between metal–pterins and HO˙ is exergonic only when pterins are bonded to Zn, Cd and Hg (cations). This new information may contribute to elucidate the way pterins participate in oxidative stress. Besides this, these results may inspire new experiments similar to those reported previously for the reaction of guanine with metal atoms.

 Please wait while we load your content...
Please wait while we load your content...