Toward preparative resolution of chiral alcohols by an organic chemical method†
Abstract
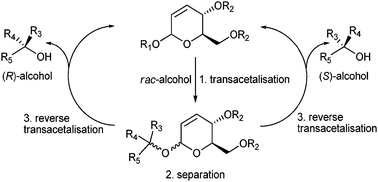
Asymmetric alcohols were resolved as 1-α-O-alkyl-2,3-unsaturated hexosides. After separation of diastereoisomers, the auxiliary and the enantiomeric alcohol were recovered by transglycosidation. Potential applications include resolution of labile secondary and

 Please wait while we load your content...
Please wait while we load your content...