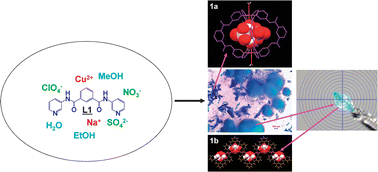
A conformationally flexible bis-pyridyl-bis-amide ligand, namely N,N′-bis-(3-pyridyl)isophthalamide (L1), has been exploited to synthesize metalla-macro-tricyclic cryptands by reacting it with various Cu(II) salts having different counteranions. Out of the five coordination compounds, namely [{Cu(μ-L1)2(H2O)2}·SO4·2H2O·X] (1a), [{Cu(μ-L1)(H2O)4}·SO4·3H2O]∝ (1b), [{Cu(μ-L1)2(H2O)2}·SiF6·CH3OH·4H2O]∝ (2), [{Cu(μ-L1)2(μ-Cl)}·ClO4]∝ (3) and [{Cu(μ-L1)2(H2O)}·(NO3)2·2H2O·X]∝ (4a) (X = disordered solvents), compounds such as 1a, 2 and 3 are metallacryptands, of which 3 is the first example of a polymeric metalla-macro-tricyclic cryptand. The effect of the conformation-dependent ligating topology and hydrogen bonding backbone of ligand L1, and counteranions on the formation of metallacryptands is discussed. Interestingly, an important anion, namely SO42−, has been separated by concomitantly crystallizing 1a and 1b from a complex mixture of anions, such as SO42−, NO3− and ClO4−, by following an in situ crystallization technique. Magnetic interactions in 3 have been investigated as a typical example. Weak antiferromagnetic coupling is observed in 3, as expected given the topology of the networks.

 Please wait while we load your content...
Please wait while we load your content...