Synthesis and biological applications of ionic triphenyltin(iv) chloride carboxylate complexes with exceptionally high cytotoxicity†
Abstract
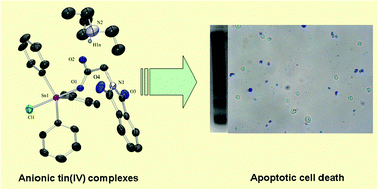
The reaction of
* Corresponding authors
a
Biozentrum, Martin-Luther-Universität Halle-Wittenberg, Weinbergweg 22, 06120 Halle, Germany
E-mail:
goran.kaluderovic@chemie.uni-halle.de
Fax: +49-345 5527028
b Institut für Chemie, Martin-Luther-Universität Halle-Wittenberg, Kurt-Mothes-Straße 2, D-06120 Halle, Germany
c
Institut für Anorganische Chemie der Universität Leipzig, Johannisallee 29, D-04103 Leipzig, Germany
E-mail:
santiago.gomez@urjc.es
Fax: +34-914888143
d Departamento de Química Inorgánica y Analítica, E.S.C.E.T., Universidad Rey Juan Carlos, 28933 Móstoles, Madrid, Spain
The reaction of

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
