Abstract
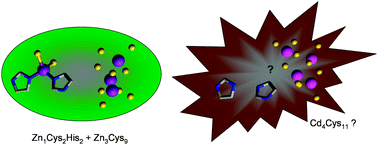
The selectivity of proteins involved in metal ion homeostasis is an important part of the puzzle to understand how cells allocate the correct metal ions to the correct proteins. Due to their similar ligand-binding properties, and their frequent co-existence in soils, essential zinc and toxic cadmium are a particularly challenging couple. Thus, minimisation of competition of Cd2+ for Zn2+ sites is of crucial importance for organisms that are in direct contact with soil. Amongst these, plants have an especially critical role, due to their importance for nutrition and energy. We have studied an embryo-specific, zinc-binding metallothionein (EC) from wheat by nuclear magnetic resonance, electrospray mass spectrometry, site-directed mutagenesis, and molecular modelling. Wheat EC exploits differences in affinities of Cys4 and Cys2His2 sites for Cd2+ and Zn2+ to achieve metal-selective protein folding. We propose that this may constitute a novel mechanism to discriminate between essential Zn2+ and toxic Cd2+.
- This article is part of the themed collection: Emerging Investigators 2010

 Please wait while we load your content...
Please wait while we load your content...