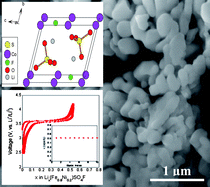
In the current scenario, Li-ion batteries are no longer limited to portable electronic devices, but are rapidly gaining momentum to enter the large-scale hybrid automotive market owing to their adequate energy density coupled with their low cost and safety. LiFePO4 is the front-runner candidate in this sector mainly due to its economic cost and operational safety. Recently, our group has discovered a novel 3.6 V metal fluorosulfate (LiFeSO4F) electrode system, which combines sulfate polyanions with fluorine chemistry to deliver excellent conductivity and electrochemical capacity. In the current study, we extend our effort to investigate the structure and electrochemical properties of 3d-transition metal (M = Co, Ni, Mn) substituted fluorosulfates. Toward this goal, we have adopted ionothermal synthesis to fabricate three families of solid-solution systems, namely Li(Fe1−xCox)SO4F, Li(Fe1−xNix)SO4F and Li(Fe1−xMnx)SO4F at temperatures as low as 300 °C. The structure, thermal stability and electrochemical properties of these mixed sulfate phases along with the end members (LiCoSO4F, LiNiSO4F and LiMnSO4F) have been examined using a suite of characterization techniques. Overall, a 3.6 V Fe2+/3+ redox reaction is observed with no signature of Co2+/3+, Ni2+/3+ or Mn2+/3+ reaction. These metal fluorosulfate systems, delivering near theoretical capacity, stand as an alternative new class of electrodes for varied commercial applications.

 Please wait while we load your content...
Please wait while we load your content...