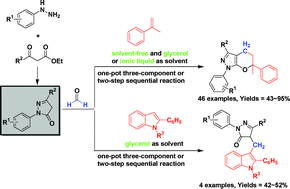
Many multicomponent reactions (MCRs) of 1,3-disubstituted 5-pyrazolones and formaldehyde were developed in environmentally benign solvent systems. Styrenes, vinylferrocene and 2-phenylindoles could easily react, under solvent-free conditions or in glycerol solvent, with 1,3-disubstituted 5-pyrazolones and paraformaldehyde in the absence of any catalyst to afford a variety of complex skeletons in moderate to excellent yields. Particularly, these MCRs are proved to be combinable with the synthesis of 1,3-disubstituted 5-pyrazolones from phenylhydrazines and β-ketone esters in glycerol or a carboxylic acid-functionalized ionic liquid, [MIm-CO2H]BF4. Therefore, some two-step sequential reactions of phenylhydrazines, β-ketone esters, formaldehyde and styrenes or indoles were developed for the first time. All these MCRs were conducted in environmentally benign solvent systems that not only minimize generation of wastes but also simplify the work-up procedure.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...