The use of ionic liquids in the synthesis of zinc imidazolate frameworks†
Abstract
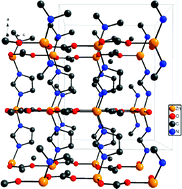
Four ![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (3) and [Zn(C3N2H3)2], I41 (4). Compounds 1 and 4 have already known structures, but have been synthesised for the first time using ionothermal methods. Compounds 2 and 3 are novel and have been synthesized for the first time in this work. The syntheses of 3 and 4 take place under almost exactly the same conditions, except that a lower synthesis temperature leads to a proportion of terminal
(3) and [Zn(C3N2H3)2], I41 (4). Compounds 1 and 4 have already known structures, but have been synthesised for the first time using ionothermal methods. Compounds 2 and 3 are novel and have been synthesized for the first time in this work. The syntheses of 3 and 4 take place under almost exactly the same conditions, except that a lower synthesis temperature leads to a proportion of terminal

 Please wait while we load your content...
Please wait while we load your content...