Development of next-generation photolabile copper cages with improved copper binding properties
Abstract
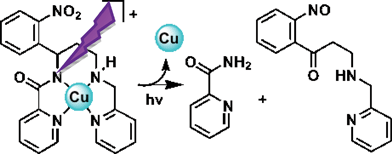
Seven new
* Corresponding authors
a
Department of Chemistry, Duke University, P.O. Box 90346, Durham, North Carolina, USA
E-mail:
Katherine.Franz@duke.edu
Seven new

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
K. L. Ciesienski, K. L. Haas and K. J. Franz, Dalton Trans., 2010, 39, 9538 DOI: 10.1039/C0DT00770F
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
