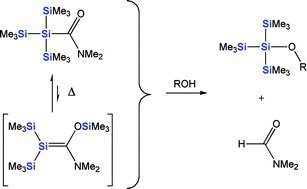
Thermolytic formation of transient 1,1-bis(trimethylsilyl)-2-dimethylamino-2-trimethylsiloxysilene (2) from N,N-dimethyl(tris(trimethylsilyl)silyl)methaneamide (1) in presence of a series of alcohols was investigated. The products are, however, not the expected alcohol-silene addition adducts but silylethers formed in nearly quantitative yields. Thermolysis of 1 in the presence of both alcohols (MeOH or iPrOH) and 1,3-dienes (1,3-butadiene or 2,3-dimethyl-1,3-butadiene) gives alkyl-tris(trimethylsilyl)silylethers and the [4+2] cycloadducts between the silene and diene, which confirms the presence of 2 and that it is unreactive towards alcohols. The observed silylethers are substitution adducts where the amide group of the silylamide is replaced by an alkoxy group, and the reaction time is reflected in the steric bulk of the alcohol. Indeed, the formation of silylethers from the reaction of alcohols with silylamide represents a new base-free method for protection of alcohols. The protection reactions using 1 progresses at elevated temperatures, or alternatively, under acid catalysis at ambient temperature, and similar protections can be carried out with N-cyclohexyl(triphenylsilyl)methaneamide and N,N-dimethyl(trimethylsilyl)methaneamide. The latter silylamide can be used under neutral conditions at room temperature. The only by-products are formamides (N,N-dimethylformamide (DMF) or N-cyclohexylformamide), and the reactions can be performed without solvent. In addition to alcohols we also examined the method for protection of diols, thiols and carboxylic acids, and also these reactions proceeded in high yields and with good selectivities.

 Please wait while we load your content...
Please wait while we load your content...