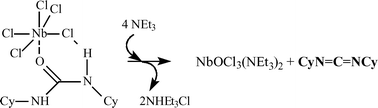
NbCl5·(N,N′-dicyclohexylurea) 1a owns a distorted octahedral structure due to intramolecular NH⋯Cl bonding. The unit cell contains four units which are intermolecularly NH⋯Cl and NH⋯N bonded. An extended intramolecular network of H-bonding (N–H⋯Cl, CH⋯Cl, CH⋯N) causes the 3D self assembling of the units. Upon addition of base, the HCl release from 1a is observed with the transfer to Nb of the O-atom of the carbonylic function of the starting urea which is converted into the relevant carbodiimide CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy 4. The latter is quantitatively released by adding an excess of NEt3 at 308 K (py and DBU are less efficient) with formation of the known NbOCl3(NEt3)2, isolated in quantitative yield. Increasing the temperature leads to a loss in selectivity as the formed DCC undergoes further reactions. At 350 K, the isocyanate CyN
NCy 4. The latter is quantitatively released by adding an excess of NEt3 at 308 K (py and DBU are less efficient) with formation of the known NbOCl3(NEt3)2, isolated in quantitative yield. Increasing the temperature leads to a loss in selectivity as the formed DCC undergoes further reactions. At 350 K, the isocyanate CyN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O has been isolated in 60% yield besides a mixture of Nb-complexes. DFT calculations have been coupled to IR and NMR experiments for characterizing possible reaction intermediates and the behaviour of 1a. Several other MClx species (ScCl3,YCl3,LaCl3,TiCl4, TaCl5, AlCl3, SnCl4) have been shown to be able to co-ordinate DCU but not all of them promote the conversion of urea into DCC.
O has been isolated in 60% yield besides a mixture of Nb-complexes. DFT calculations have been coupled to IR and NMR experiments for characterizing possible reaction intermediates and the behaviour of 1a. Several other MClx species (ScCl3,YCl3,LaCl3,TiCl4, TaCl5, AlCl3, SnCl4) have been shown to be able to co-ordinate DCU but not all of them promote the conversion of urea into DCC.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCy 4. The latter is quantitatively released by adding an excess of
NCy 4. The latter is quantitatively released by adding an excess of ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O has been isolated in 60% yield besides a mixture of
O has been isolated in 60% yield besides a mixture of 

 Please wait while we load your content...
Please wait while we load your content...