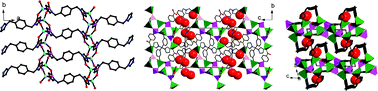
This paper reports mixed ligated metal phosphonates with formula [Zn2(4-cppH)2(L1)] (1), [Zn3(4-cpp)2(L2)2]·2.5H2O (2), [Zn3(4-cpp)2(L3)]·H2O (3) and [Ni2(4-cppH)2(L1)3]·2H2O (4), where L1 = 1,4-bis(imidazol-1-ylmethyl)benzene, L2 = 1,3-bis(imidazol-1-ylmethyl)-benzene, L3 = 1,2-bis(imidazol-1-ylmethyl)benzene and 4-cppH3 = 4-carboxyphenylphosphonic acid. Compound 1 shows a layer structure in which the inorganic double chains made up of {ZnO3N} and {PO3C} tetrahedra are connected by L1 bridges in a trans-mode. In compound 2, an inorganic hexanuclear cluster is observed where the six zinc atoms are bridged purely through O–P–O units from four 4-cpp3−. The L2 ligand adopts both cis- and trans-coordination modes to link the hexanuclear clusters into a three-dimensional framework structure with primitive cubic pcu topology. Compound 3 displays another type of layer structure in which the inorganic chains made up of {ZnO3N} (or {ZnO4}) and {PO3C} tetrahedra are linked by both the 4-cpp3− and L3 ligands. The L3 ligand adopts cis-coordination mode. Unlike Zn(II) ions in 1–3, the Ni(II) ions in compound 4 are octahedrally coordinated, and are bridged by both 4-cppH2− and L1 ligands, resulting in an interesting double layer structure with the lattice water filling in the intralayer space. The thermal stabilities, luminescent and magnetic properties are investigated.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...