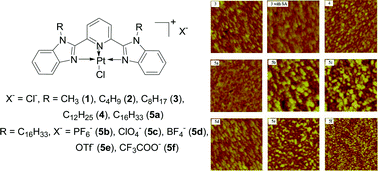
A systematic photophysical study, i.e. electronic absorption and emission study, on platinum(II) 2,6-bis(N-alkylbenzimidazol-2′-yl)pyridine complexes with different alkyl chains and different anions were performed in solutions and in Langmuir–Blodgett (LB) films. Electronic absorption in solution shows high-energy bands that are assigned to π,π* transitions within the ligand and a moderately intense metal-to-ligand charge transfer (1MLCT) band above 400 nm. All the complexes are emissive at room temperature and at 77 K. When low-polarity solvent, such as hexane was added to the CHCl3 solutions of these complexes, solvent-induced aggregation occurred, which was evident by the appearance of a broad metal–metal-to-ligand charge transfer (1MMLCT) band between 500 and 600 nm in their UV-vis spectra and a new emission band appearing around 670 nm, which is attributed to the 3MMLCT state. The degree of aggregation is affected drastically by the nature of the counter anion, with complexes 5b, 5c and 5d that contain PF6−, ClO4− and BF4− anion, respectively, appearing to more readily aggregate. In contrast, the length of the alkyl chain shows a negligible effect on the degree of aggregation. The emission lifetime measurements further confirm the formation of aggregates. These complexes exhibited selective vapochromic response when exposed to volatile organic compounds (VOCs). Both the alkyl chain length and the counter anion affect the vapochromic effect. Moreover, LB films were fabricated using these amphiphilic platinum complexes and most of the films displayed 3MMLCT emission. The emission energy and the lifetime in LB films are influenced significantly by the counter anions. Complex 5f mainly emits from a ligand–ligand π–π interacted excited state, and 5a–5e exhibit a 3MMLCT emission. Although no simple systematic correlation could be made between the nature of the counter ion and the electronic absorption and/or emission properties of these platinum complexes in solution and in LB films, we believe that the salient counter ion effects are most likely related to the size and shape of the counter ions.

 Please wait while we load your content...
Please wait while we load your content...