In the η3-butadienyl complex Ru{η3-C(CN)2CPhC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 1, which is formed from Ru(C
C(CN)2}(PPh3)Cp 1, which is formed from Ru(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)(PPh3)2Cp and tcne, a CN group reacts with MeO− to give the methoxy-amide Ru{NH
CPh)(PPh3)2Cp and tcne, a CN group reacts with MeO− to give the methoxy-amide Ru{NH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)C(CN)
C(OMe)C(CN)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCPh
CCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 2, in which the NH has displaced the C
C(CN)2}(PPh3)Cp 2, in which the NH has displaced the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C from the Ru centre with formation of a RuC3N ring. “Click addition” of azide to a CN group in 1 gives the oligomeric tetrazolato complex Ru{N3N[Na(OEt2)]
C from the Ru centre with formation of a RuC3N ring. “Click addition” of azide to a CN group in 1 gives the oligomeric tetrazolato complex Ru{N3N[Na(OEt2)]![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(CN)
CC(CN)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCPh
CCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 3, also containing a RuC3N ring. Salt-elimination reactions of 3 with MeOTf, FeCl(dppe)Cp, RuCl(dppe)Cp* and trans-PtCl2{P(tol)3}2 result in selective substitution at one nitrogen atom of the RuC3N ring. Geometries of 1 and the anion in 3 were computed by DFT methods. Preferences for CN groups attacked in the nucleophilic and cycloaddition reactions of 1 are supported by NBO calculations. Alkylation of 1 in reactions with 1,2-dimethoxyethane gave two isomers of Ru{N3[CH(CH2OMe)(OMe)]N
C(CN)2}(PPh3)Cp 3, also containing a RuC3N ring. Salt-elimination reactions of 3 with MeOTf, FeCl(dppe)Cp, RuCl(dppe)Cp* and trans-PtCl2{P(tol)3}2 result in selective substitution at one nitrogen atom of the RuC3N ring. Geometries of 1 and the anion in 3 were computed by DFT methods. Preferences for CN groups attacked in the nucleophilic and cycloaddition reactions of 1 are supported by NBO calculations. Alkylation of 1 in reactions with 1,2-dimethoxyethane gave two isomers of Ru{N3[CH(CH2OMe)(OMe)]N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(CN)
CC(CN)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCPh
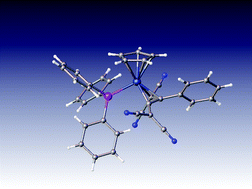
CCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 8 and 9, differing in the sites of attachment of the alkyl group, likely by radical processes. The molecular structures of eight complexes are reported, including a re-determination of 1. Computed NMR chemical shifts are used to reassign the butadienyl carbon resonances in the 13C NMR spectrum of 1.
C(CN)2}(PPh3)Cp 8 and 9, differing in the sites of attachment of the alkyl group, likely by radical processes. The molecular structures of eight complexes are reported, including a re-determination of 1. Computed NMR chemical shifts are used to reassign the butadienyl carbon resonances in the 13C NMR spectrum of 1.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 1, which is formed from
C(CN)2}(PPh3)Cp 1, which is formed from ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CPh)(PPh3)2Cp
CPh)(PPh3)2Cp![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(OMe)C(CN)
C(OMe)C(CN)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCPh
CCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 2, in which the
C(CN)2}(PPh3)Cp 2, in which the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C from the
C from the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(CN)
CC(CN)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCPh
CCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 3, also containing a RuC3N ring.
C(CN)2}(PPh3)Cp 3, also containing a RuC3N ring. ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CC(CN)
CC(CN)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CCPh
CCPh![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CN)2}(PPh3)Cp 8 and 9, differing in the sites of attachment of the
C(CN)2}(PPh3)Cp 8 and 9, differing in the sites of attachment of the 

 Please wait while we load your content...
Please wait while we load your content...