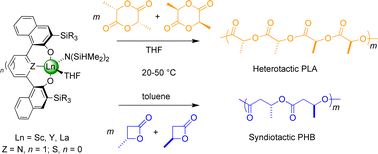
Scandium, yttrium and lanthanum amido complexes supported by tridentate bis(ortho-silyl-substituted naphtholate)-donor ligands ({ONOSiPh3}2− and {ONOSitBuMe2}2−, donor = 2,6-pyridine; {OSOSiPh3}2−, donor = 2,5-thiophene) have been prepared in high yields (72–96%) by reaction of the corresponding pro-ligand {OZOSiR3}H2 and Ln[N(SiHMe2)2]3(THF)n precursor. The solid-state structures of {ONOSiPh3}La[N(SiHMe2)2](THF) (3), {ONOSitBuMe2}Ln[N(SiHMe2)2](THF) (Ln = Sc, 4; Y, 5) and {OSOSiPh3}Ln[N(SiHMe2)2](THF) (Ln = Sc, 7; La, 9) have been determined by single-crystal X-ray diffraction studies. In all five complexes, the naphtholate rings twist in the same direction from the plane of the pyridine or thiophene linker, to give rise to Cs-symmetric (non crystallographic) structures. Compounds 1–9 are single-site initiators for the ring-opening polymerization (ROP) of racemic lactide (rac-LA) at 20 °C, affording poly(lactides)s (PLAs) with relatively narrow polydispersities and molecular weights in good agreement with calculated values. When carried out in THF, the polymerizations afforded heterotactic-enriched PLAs (Pr up to 0.93), while atactic polymers are formed in toluene. Compounds 1–3 and 7–9, having o-SiPh3 substituents on the naphtholate rings, are also active for the ROP of racemicβ-butyrolactone at 20–50 °C, to form syndiotactic-enriched poly(3-hydroxybutyrate)s (PHBs) (Pr up to 0.87) when using toluene as the solvent, whereas atactic polymers were obtained in THF. The nature of the metal center (Sc, Y, La), the central linker in the ligand framework (pyridine, thiophene), and the ortho-silyl substituent (SiPh3, SiMe2tBu) significantly affect the degree of stereocontrol in those polymerizations.

 Please wait while we load your content...
Please wait while we load your content...