Disproportionation and radical formation in the coordination of “GaI” with bis(imino)pyridines†
Abstract
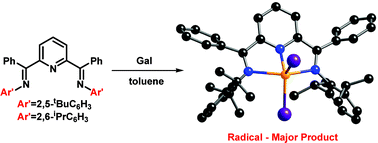
Attempted coordination of “GaII” with two new sterically bulky, aryl substituted ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3)]GaI2+GaI4− (
CPh}2(NC5H3)]GaI2+GaI4− (![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3)]GaI2. Both
CPh}2(NC5H3)]GaI2. Both ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3) with
CPh}2(NC5H3) with ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CPh}2(NC5H3)]AlCl2+
CPh}2(NC5H3)]AlCl2+

 Please wait while we load your content...
Please wait while we load your content...