Hydrogen bond assisted co-crystallization of a bimetallic MnIII2NiII2cluster and a NiII2cluster unit: synthesis, structure, spectroscopy and magnetism†
Abstract
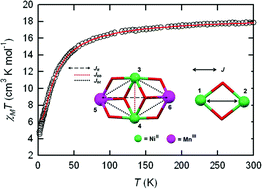
A new bimetallic Schiff-base composite complex [Ni2(LH2)2(H2O)2Cl2][Mn2Ni2(LH)4]2Cl4(CH3OH) (1) has been synthesized by a simple one-pot reaction. The compound was structurally characterized by
- This article is part of the themed collection: Molecular magnets

 Please wait while we load your content...
Please wait while we load your content...