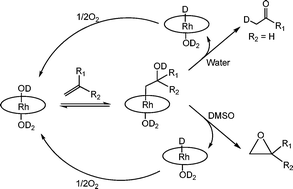
Reactivity and kinetic–mechanistic studies of regioselective reactions of rhodium porphyrins with unactivated olefins in water that form β-hydroxyalkyl complexes and conversion to ketones and epoxides†
Abstract
This article reports on the selective
- This article is part of the themed collection: New Talent: Inspiration for the future

 Please wait while we load your content...
Please wait while we load your content...