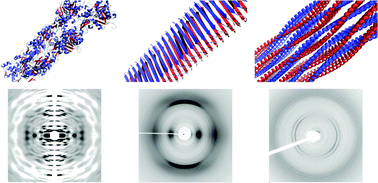
Protein and peptide self-assembly is integral to a growing number of misfolding diseases that involve the ordered assembly and deposition of misfolded proteins as amyloid fibrils. However, it is becoming clear that these highly stable assemblies can be exploited as potential bionanomaterials. Recent work reveals that amyloid-like assembly is carefully controlled by a number of organisms to form functional amyloid. It is essential to gain an understanding of how sequence influences the assembly mechanism, as well as the final structure, to enable the control that is required to fully exploit these materials. In this tutorial review, we will discuss the different types of fibrous protein assembly from structural and cytoskeletal proteins through to misfolded proteins and finally designed self-assembling peptides. In the second part, we will discuss the advances in structure determination, with particular reference to the use of X-ray fibre diffraction.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...