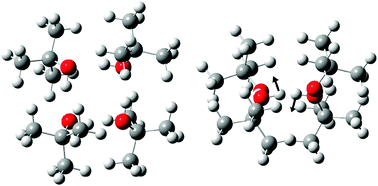
The benefits of alternation and alkylation: large amplitude hydrogen bond librational modes of alcohol trimers and tetramers†
Abstract
Intermolecular
- This article is part of the themed collection: Chemical Dynamics of Large Amplitude Motion

 Please wait while we load your content...
Please wait while we load your content...