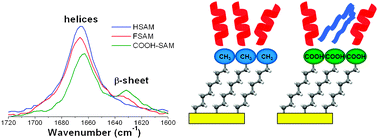
Ion soft landing (SL) enables highly selective modification of substrates for applications in materials science, nanotechnology and biology. Our previous study [P. Wang and J. Laskin, Angew. Chem., Int. Ed., 2008, 47, 6678–6680] showed that SL can be used for preparation of conformation-selected peptide arrays. Here we present a first study of the effect of the surface on the secondary structures of peptides soft-landed onto self-assembled monolayer surfaces (SAMs). Conformations of soft-landed peptide ions were examined using a newly constructed instrument that enables in situ infrared reflection absorption spectroscopy (IRRAS) characterization of surfaces during and after ion deposition. Polyalanine peptides, Ac-AnK and Ac-KAn (n = 7, 15), that have been extensively studied both in solution and in the gas phase were used as model systems in this study. We demonstrate that physical and chemical properties of SAM surfaces have a strong effect on the conformations of soft-landed peptide ions. For example, deposition of the α-helical [Ac-A15K + H]+ ion on the CH3-terminated (HSAM) surface results in immobilization of both the α- and 310-helical conformations. In contrast, a significant fraction of Ac-A15K molecules are present in the β-sheet conformation on the CF3-(FSAM) and COOH-terminated (COOH-SAM) surfaces. We show that the kinetic energy of the polyalanine ion, the charge, and the initial conformation have only a minor effect on the conformation of deposited species suggesting that the interaction between the molecule and the surface plays a major role in determining the secondary structures of immobilized polyalanines. This study demonstrates that SL of mass-selected ions can be utilized for obtaining fundamental understanding of the intrinsic properties of biomolecules and surfaces responsible for conformational changes upon adsorption.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...