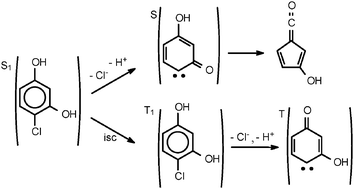
Research on photoinduced reactions of halogenated phenols is of interest for environmental photochemistry and for synthetic organic chemistry. Previous studies have uncovered interesting mechanistic features, including ring contraction from benzene to cyclopentadiene from ortho derivatives and two-electron processes forming carbocations and carbenes from para derivatives. In the present work, we studied the aqueous photochemistry of 4-chlororesorcinol (1), which combines the conformational properties of both types of derivatives, using nanosecond transient absorption spectroscopy and photoproduct analysis. The absorption spectra obtained upon pulsed laser excitation of 1 showed the occurrence of both o-Cl and p-Cl elimination, the first observed transients being the ketene 3-hydroxy-6-fulvenone (2, λmax = 255 nm) and the carbene 2-hydroxy-4-oxo-2,5-cyclohexadienylidene (3, λmax = 405 and 395 nm). The reactivities of 2 and 3, the spectra of the secondary transients and the analysis of the final products showed that the two HCl elimination pathways take place concurrently. Most probably, the bifurcation step is the competition between intersystem crossing on the molecular level and o-Cl elimination on the singlet surface; p-Cl elimination proceeds on the triplet surface. Remarkably, the quantum yield of p-Cl elimination from 1 is lower by one order of magnitude compared to that found in para-halogenated phenols, while that of o-Cl elimination from 1 is comparable to ortho-halogenated phenol. To explain this result, we propose that o-Cl elimination is the major deactivation step, forming an intermediate singlet cation which is able to recombine to ground state 1, thereby limiting the observed photochemical quantum yields.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...