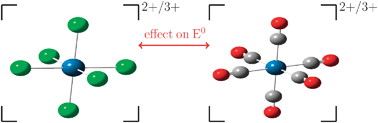
Adiabatic energy differences ΔEadiabatic are computed at density functional theory (DFT) level for the oxidation half reaction of [M(CO)nL6−n] complexes (M = Ru2+/3+, Os2+/3+, Tc2+/3+; L = CN−, Cl−, water, CH3CN, N2 and CO). Linear trends in ΔEadiabatic with respect to the substitution number n(CO) support the hypothesis of additive ligand effects on the redox potential. The values of the slope of these linear regression curves are shown to be independent of metal type (Ru, Os and Tc) and can therefore act as a ligand specific parameter. Based on these parameters, a computed electrochemical series was constructed, which was in good agreement with Pickett's PL, Lever EL(L) and CEP parameters. The linearity in ΔEadiabatic is also reflected in the structural properties such as the M–CO bond distances of [M(CO)nL6−n] complexes. An energy decomposition analysis of the bond between the metal fragment and ligand gave an additional insight into the ligand's bonding properties in terms of electrostatic and orbital contributions.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...