Formation dynamics and nature of tryptophan's primary photoproduct in aqueous solution†
Abstract
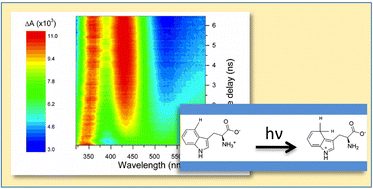
The excited state quenching and photoproduct formation of
- This article is part of the themed collection: Quantum molecular dynamics and control

 Please wait while we load your content...
Please wait while we load your content...