The interface bonding and orientation of a quinonoid zwitterion†
Abstract
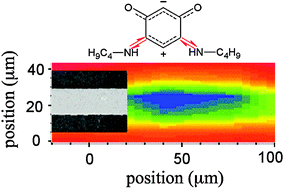
We have investigated the interaction and orientation of a strongly dipolar zwitterionic ![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) NHR)2(
NHR)2(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O)2 where R = n-C4H9, on both
O)2 where R = n-C4H9, on both
* Corresponding authors
a
Dept. of Physics and Astronomy, Nebraska Center for Materials and Nanoscience, University of Nebraska-Lincoln, Lincoln NE 68588, USA
E-mail:
pdowben1@unl.edu, agruverman2@unl.edu
b Dept. of Physics, Box 8202, North Carolina State University, Raleigh, USA
c
Laboratoire de Chimie de Coordination, Institut de Chimie (UMR 7177 CNRS), Université de Strasbourg, 4 rue Blaise Pascal, 67081 Strasbourg France
E-mail:
braunstein@unistra.fr
d
Institut de Physique et Chimie des Matériaux de Strasbourg, IPCMS (UMR 7504), Université Strasbourg, 23 rue du Lœss B.P. 20, 67034 Strasbourg, France
E-mail:
bernard.doudin@ipcms.u-strasbg.fr
e The J. Bennett Johnston Sr. Center for Advanced Microstructures and Devices, Louisiana State University, 6980 Jefferson Hwy., Baton Rouge, USA
f Dept. of Physics and Electronics, Univ. of Puerto Rico-Humacao, 100 Road 908 CUH Station, Humacao, PR 00791, USA and the Institute for Functional Nanomaterials, Univ. of Puerto Rico, Facundo Bueso Bldg., Rio Piedras, USA
g Paul Scherrer Institut, Swiss Light Source, CH-5232 Villigen, Switzerland
We have investigated the interaction and orientation of a strongly dipolar zwitterionic ![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) NHR)2(
NHR)2(![[horiz bar, triple dot above]](https://www.rsc.org/images/entities/char_e0f1.gif) O)2 where R = n-C4H9, on both
O)2 where R = n-C4H9, on both

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. Xiao, Z. Zhang, D. Wu, L. Routaboul, P. Braunstein, B. Doudin, Y. B. Losovyj, O. Kizilkaya, L. G. Rosa, C. N. Borca, A. Gruverman and P. A. Dowben, Phys. Chem. Chem. Phys., 2010, 12, 10329 DOI: 10.1039/C003996A
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
