The behaviour of hydrogen in both defect free and acceptor doped bulk rutile TiO2 is investigated through defect calculations performed within the density functional theory formalism. Both interstitial and substitutional hydrogen defects are shown to behave as shallow donors in the material, thereby existing as effectively positive hydroxide defects, 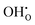 , and substitutional hydrogen defects,
, and substitutional hydrogen defects, 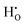 . Of the investigated isolated hydrogen defects, the
. Of the investigated isolated hydrogen defects, the 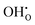 defect is shown to have the lowest formation energy over the whole Fermi level range under both oxidising and reducing conditions. However, the formation energy of
defect is shown to have the lowest formation energy over the whole Fermi level range under both oxidising and reducing conditions. However, the formation energy of 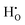 is only 0.35 eV higher under conditions where TiO2 is in equilibrium with Ti2O3, indicating that it exists as a minority defect in rutile TiO2 under highly reducing conditions such as under growth of TiO2 on a Ti metal surface. The enthalpy of hydration of oxygen vacancies is calculated to be −1.64 and −1.60 eV for the 2 × 2 × 3 and 3 × 3 × 3 supercells, indicating that
is only 0.35 eV higher under conditions where TiO2 is in equilibrium with Ti2O3, indicating that it exists as a minority defect in rutile TiO2 under highly reducing conditions such as under growth of TiO2 on a Ti metal surface. The enthalpy of hydration of oxygen vacancies is calculated to be −1.64 and −1.60 eV for the 2 × 2 × 3 and 3 × 3 × 3 supercells, indicating that 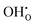 defects will prevail in wet atmospheres, even at high temperatures. Hydrogen incorporated in the material can furthermore be expected to associate significantly with various acceptor centres (e.g., substitutional N and Al acceptors and Ti vacancies). The binding energies of the substitutional NH×O (imide) defect and of the association complexes between H and fully ionised substitutional Al,
defects will prevail in wet atmospheres, even at high temperatures. Hydrogen incorporated in the material can furthermore be expected to associate significantly with various acceptor centres (e.g., substitutional N and Al acceptors and Ti vacancies). The binding energies of the substitutional NH×O (imide) defect and of the association complexes between H and fully ionised substitutional Al, 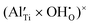 , and Ti vacancies,
, and Ti vacancies, 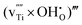 and
and 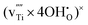 (i.e., Ruetschi type defects), are calculated to be −0.36, −0.12, −0.47 and −0.82 eV, respectively.
(i.e., Ruetschi type defects), are calculated to be −0.36, −0.12, −0.47 and −0.82 eV, respectively.
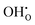 , and substitutional
, and substitutional 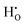 . Of the investigated isolated
. Of the investigated isolated 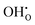 defect is shown to have the lowest formation energy over the whole Fermi level range under both oxidising and reducing conditions. However, the formation energy of
defect is shown to have the lowest formation energy over the whole Fermi level range under both oxidising and reducing conditions. However, the formation energy of 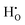 is only 0.35 eV higher under conditions where TiO2 is in equilibrium with Ti2O3, indicating that it exists as a minority defect in
is only 0.35 eV higher under conditions where TiO2 is in equilibrium with Ti2O3, indicating that it exists as a minority defect in 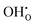 defects will prevail in wet atmospheres, even at high temperatures.
defects will prevail in wet atmospheres, even at high temperatures. 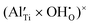 , and
, and 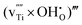 and
and 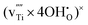 (i.e., Ruetschi type defects), are calculated to be −0.36, −0.12, −0.47 and −0.82 eV, respectively.
(i.e., Ruetschi type defects), are calculated to be −0.36, −0.12, −0.47 and −0.82 eV, respectively.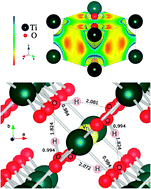

 Please wait while we load your content...
Please wait while we load your content...