Silyloxyazadienes: one intermediate and two competitive pericyclic reactions†
Abstract
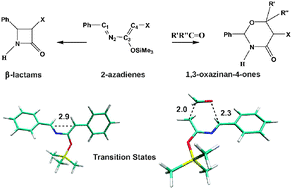
The two competing mechanisms in the reaction of 3-trialkylsilyloxy-2-aza-1,3 dienes to form β-lactams through a [2+2] electrocyclic ring closure or tetrahydrooxazinan-4-ones via a [4+2] hetero-Diels–Alder reaction were studied using Density Functional computations. Although the [2+2] and [4+2] mechanisms are typical of dienes, their competition, starting from the same diene intermediate, has not yet been observed and analyzed. This competition is governed by a delicate interplay between temperature and substituents at the diene and dienophile, respectively. Clearly, entropy tends to favor the [4+2] hetero-Diels–Alder at low temperatures and the [2+2] electrocyclic ring closure at high temperatures, but simple substituent modifications at the diene and dienophile, can make the [4+2] competitive at high temperatures and sometimes even transform the [4+2] concerted mechanism into a two-step Mukaiyama-type process. Moreover, a study of the global electrophilicity values showed that charge transfer in the hetero-Diels–Alder transition states is driven by chemical hardness rather than by chemical potential.
- This article is part of the themed collection: Molecular mechanisms of the photostability of life

 Please wait while we load your content...
Please wait while we load your content...